When two elements A and B form more than one compounds, the masses of element B that combine with a given mass of A are always in the ratio of small whole numbers this is known as law of multiple proportions .
For example, Hydrogen combines with oxygen to form two compounds, namely water and hydrogen peroxide.
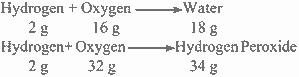
Here, it is found that, the two masses of oxygen i.e. 16 g and 32 g which combine with a fixed mass of hydrogen (2g) are in the ratio of small whole numbers, i. e. 16:32 or 1:2.