i. When a gas is collected over water in a closed container, it gets mixed with the saturated water vapour in that space. Therefore, the measured pressure corresponds to the pressure of the mixture of that gas and the saturated water vapour in that space.
ii. Pressure of pure and dry gas can be calculated by using the aqueous tension. It is obtained by subtracting the aqueous tension from the total pressure of the moist gas.
∴ PDry gas = PTotal – Paq
i.e., PDry gas = PTotal – Aqueous Tension
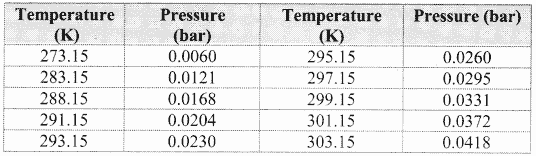
Note: Aqueous tension of water (vapour pressure) as a function of temperature.