Correct option is A) B2H6 is Lewis Acid
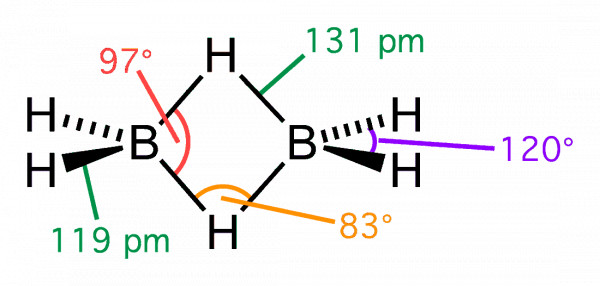
The four terminal hydrogen atoms and
the two boron atoms lie in one plane. Above and below this plane, there are two bridging
hydrogen atoms. The four terminal B-H bonds are regular two centre-two electron bonds
while the two bridge (B-H-B) bonds are different and can be described in terms of three