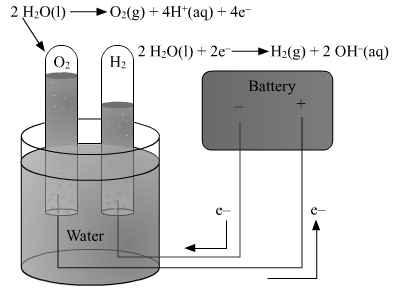
Electrolysis of water :
When electric current is passed through water containing a few drops of an acid or a few drops of a base, it dissociates to give H+ ions and OH-ions. Hydrogen gas is formed near the cathode and oxygen gas near the anode. This is called electrolysis of water.
The following equation represents the electrolysis of water :H2O(l)
In pure water, at the negatively charged cathode, a reduction reaction takes place, with electrons (e−) from the cathode being given to hydrogen cations to form hydrogen gas.
Reduction at cathode: 2 H+ + 2e− → H2
On positively charged anode, an oxidation reaction occurs, generating oxygen gas by giving electrons to the anode :
Oxidation at anode: 2 H2O → O2(g) + 4 H+(aq) + 4e−
Overall reaction: 2 H2O(l) → 2 H2(g) + O2(g)
The number of hydrogen molecules produced is thus twice the number of oxygen molecules. The produced hydrogen gas has therefore twice the volume of the produced oxygen gas. The number of electrons pushed through the water is twice the number of generated hydrogen molecules and four times the number of generated oxygen molecules.