The structure of CH3Cl is given below:
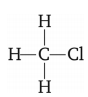
Carbon has four valence electrons. It shares 1 electron each with 3 hydrogen atoms and 1 electron with chlorine. The bond between C and Cl atoms is covalent but due to higher value of electro-negativity of Cl, the C–Cl bond is polar in nature.