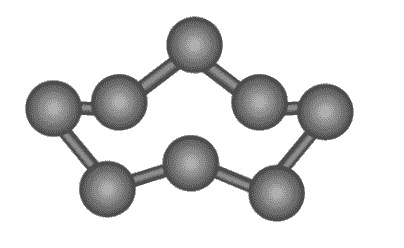
Sulphur has an atomic number of 16 and an electronic configuration of 2,8,6. The valance electrons on the sulphur atom are six. The sulphur molecule’s chemical formula is S 8, and each sulphur atom is connected to identical atoms on either side by single covalent bonds, completing its octet .